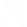The Sigray QuantumLeap X-Ray Absorption Spectroscopy System – Chemical Specification and Electronic Structure with X-Ray Absorption Spectroscopy. We answer your questions in a series of articles about the QuantumLeap.
See also:
What are the relative advantages of the QuantumLeap XAS compared to existing laboratory XAS systems?
Although previous laboratory XAS systems have been able to perform EXAFS on high concentration samples e.g. pure Al/Fe foil, they are unable to achieve weight percentages of <10%. Moreover, these systems cannot operate below 4-5keV X-ray energies whilst the QuantumLeap can operate as low down as 2.1keV (which allows for studies of elements like Phosphorous) with lower energies difficult to achieve in a non-UHV (10-9 Torr) environment due to being absorbed by air easily.
What is the difference between XANES and EXAFS?
XANES (ranging from up to 30-40eV), acquired in an off-Rowland geometry, require high energy resolution whilst EXAFS (ranging from 100eV – 1000eV), acquired in a von Hamos geometry, require lower energy resolution but higher flux.
Whilst XANES provide internal information on an atom, including oxidation state, EXAFS provide nearest neighbouring atom information through single (NEXAFS) and multiple scatt including interatomic distance, bonding and local structure.
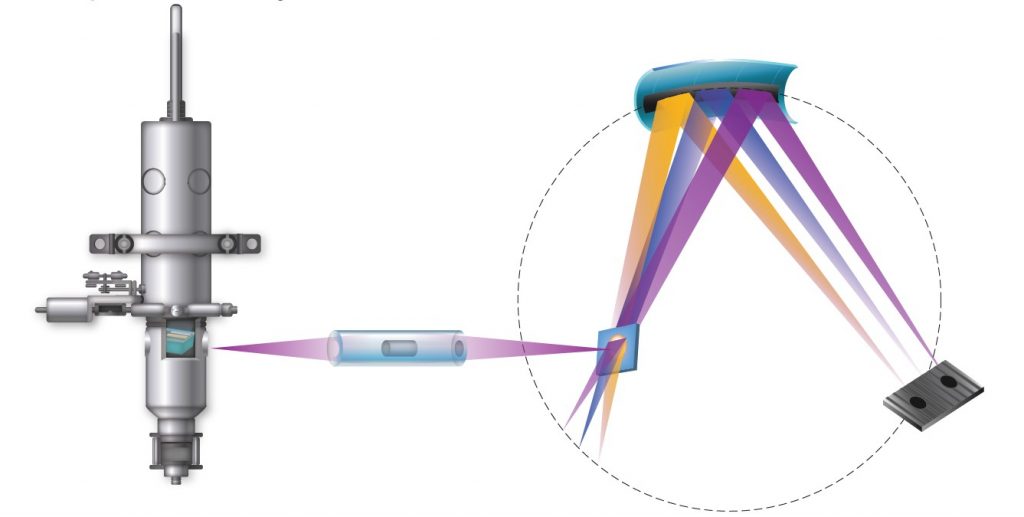


What is the difference between XAS and XES?
XAS is based upon information from transmitted x-rays, whereas XES is based upon information from emitted x-rays. In the XAS setup, the detector is placed on the opposite side to the source whilst, in XES, the detector is located on the same side as the x-ray source.
What is the difference between XAS and XPS?
XAS is based upon x-ray excitation; XPS relies on the photoelectric effect and thus electron excitation. As such, XAS is not surface sensitive whilst XPS is (~10nm) and so does not require a UHV environment. This means that XAS allows for the study of a wider range of samples e.g. resins, polymers etc… with minimal sample preparation and allowing for possible in-situ studies. Furthermore, charging (resulting in peak shifting) is also a phenomena not present in XAS spectra as opposed to XPS.
However, quantification with XPS is generally easier to perform and lighter elements (with exception of H, Li (unless in extremely high concentrations) and B (unless in very high concentrations) are easier to detect.